The Pharmacy and Poisons Board (PPB) has cautioned Kenyans against using Ozempic Pens (Semaglutide) to treat type 1 and type 2 diabetes.
In a statement on Thursday, July 18, PPB said it had received an alert from Interpol that the Apidra Solostar pens (glulisine) have been relabeled as Ozempic (Semaglutide) Pens.
The board noted that the Ozempic pens are not authorized in the Kenyan market and any product marketed as such is illegal in the market.
“Consequently, the Board would like to inform the public that Ozempic Pens are currently not registered or authorized by the Board to be placed in the Kenyan market. Therefore, any product being marketed as Ozempic Pens is illegally in the market and the Board cannot ascertain their safety, quality and effectiveness,” read the statement in part.
The board noted that it has initiated a rapid response and heightened surveillance to verify whether the falsified Ozempic Pens are presently circulating in the Kenyan market.
Read More
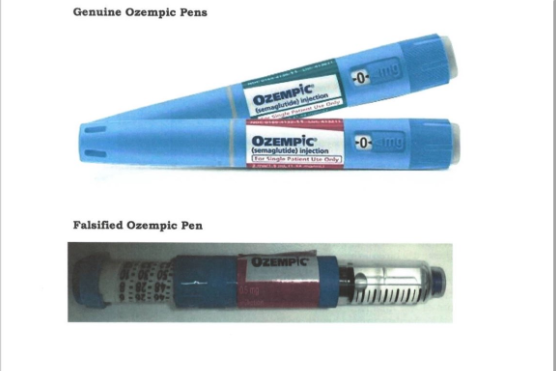
Further, PPB cautioned the public and healthcare professionals against trading, distributing, wholesaling, retailing, issuing, dispensing, using, or administering counterfeit Ozempic Pens to patients.
“The Board remains committed to protecting the health of the public and encourages the public to remain vigilant at all times and promptly report any suspected cases of sub-standard/falsified health products or adverse drug reactions,” the board stated.
Additionally, PPB called on members of the public and healthcare professionals to share any information regarding Ozempic pens with the board.




