The Pharmacy and Poisons Board (PPB) has ordered an immediate quarantine of Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017, a drug used for the management of cancer.
In a statement on Thursday, December 5, the poisons board disclosed that the order was made due to complaints made on the appearance of the content of the drug.
"The Pharmacy and Poisons Board orders the quarantine of Flurasted 500 (5-Fluorouracil) Injection Batch No. HHP24017 Manufactured by Halsted Pharma Private Limited, India.
"The quarantine order is being issued due to a market complaint on the appearance parameter of the content," PPB CEO Fred Siyoi remarked.
Siyoi directed all pharmaceutical outlets and health facilities to quarantine the product batch and stop further distribution and use of the affected batch.
Read More
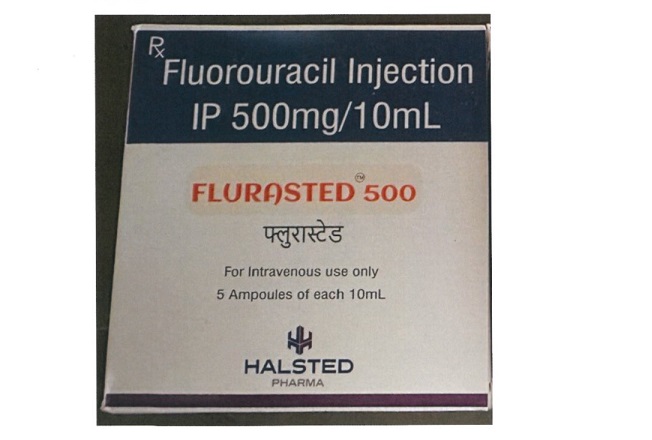
“In light of this, the Board advises all pharmaceutical outlets, healthcare facilities, healthcare professionals, and members of the public to immediately quarantine the product batch and stop the further distribution, sale, issuance, or use of the affected batch,” he stated.
The PPB CEO urged Kenyans to report any suspected cases of substandard medicines or adverse drug reactions to the nearest healthcare facility or the poisons board.
The order comes a few weeks after PPB gave another order to recall a drug due to labelling mix-ups during the packing process.
''The Pharmacy and Poisons Board has mandated the recall of Efinox 1% w/v Batch No. 82979 and Efinox 0.5% w/v Batch No. 82978 manufactured by Laboratory and Allied Ltd, Kenya. The recall is being issued due to labeling mix-ups where the correct product was identified but the wrong strength was applied," PPB stated on November 22.


-1770368133.png)




