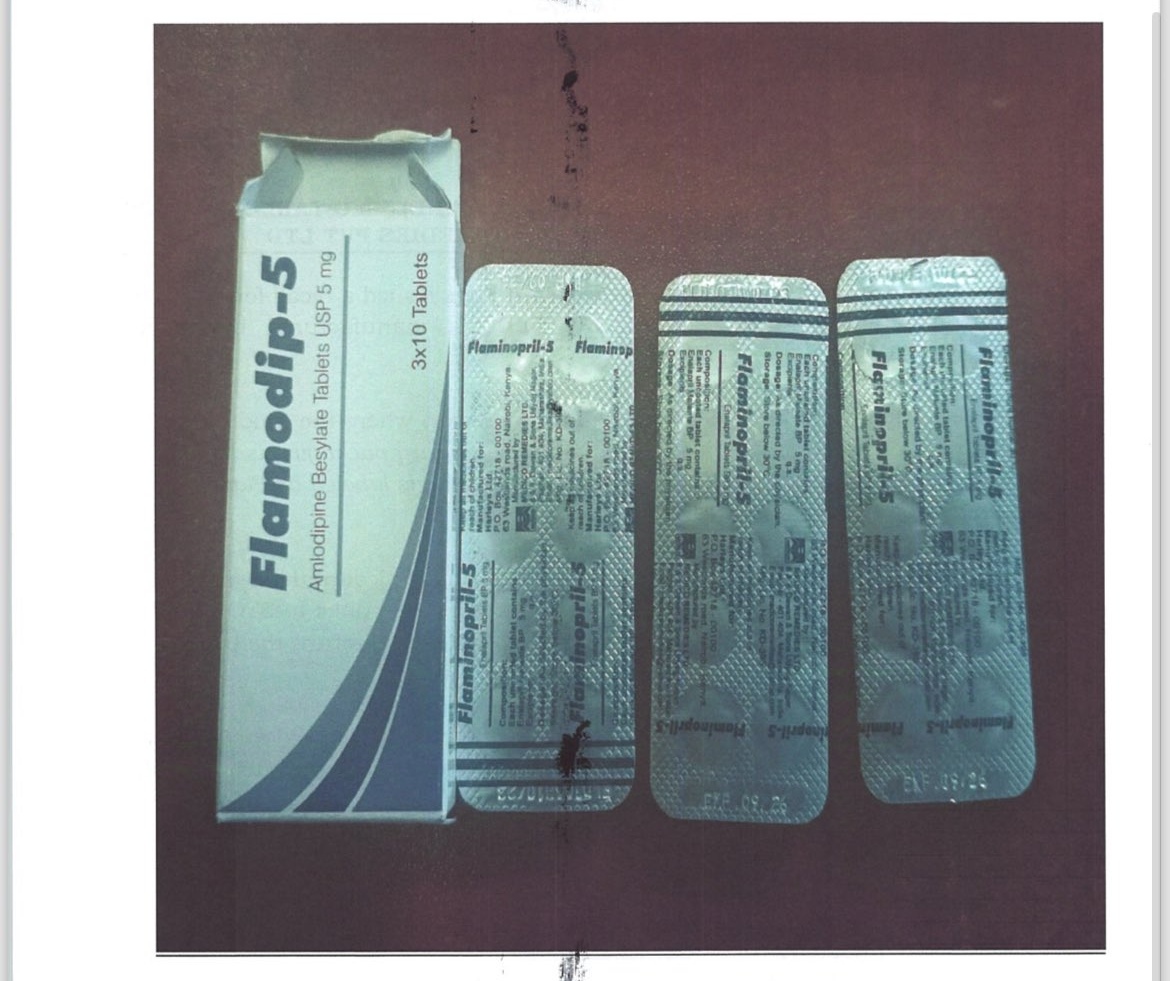
The Pharmacy and Poisons Board has issued a recall for Flamodip (Amlodipine) 5mg Tablets, Batch No. FLD303, manufactured by Medico Remedies Pvt Ltd.
In a statement released on Friday, September 27, the board indicated that the measure was necessitated by a labeling error that did not correctly represent the contents of the medication.
"The secondary packaging is labeled as Flamodip-5 (Amlodipine), while the primary packaging is labeled as Flaminopril-5 (Enalapril)," PPB said in the statement.
As such, the board has advised pharmaceutical outlets and healthcare facilities to stop the distribution and issuance of the product batch.
"Stop further distribution, sale, issuing, or use of the product batch and return the product to their nearest healthcare facility or respective suppliers. The Board encourages the public to promptly report any suspected cases of substandard medicines or adverse drug reactions to the nearest healthcare facility or the Pharmacy and Poisons Board," the statement further read.
Read More
Flamodip, containing Amlodipine, is a calcium channel blocker primarily used to treat hypertension and chronic stable angina.
By inhibiting calcium influx into cardiac and vascular smooth muscle cells, it promotes vasodilation, thereby reducing blood pressure and myocardial oxygen demand.
Flamodip is typically prescribed for patients with high blood pressure, coronary artery disease, and angina.
It can be used alone or in combination with other medications like beta-blockers or ACE inhibitors to enhance therapeutic effects.
Common side effects include swelling, fatigue, and abdominal pain, while serious risks may involve low blood pressure or heart attack.