The Ministry of Health has moved to address reports regarding the alleged sale of a newly introduced injectable HIV prevention drug in public health facilities.
In a statement on Thursday, February 19, the National AIDS & STI Control Programme (NASCOP) clarified that the long-acting injectable pre-exposure prophylaxis (PrEP) drug, Lenacapavir, will not be sold but will instead be provided at no cost.
"The long-acting Injectable HIVnPre-Exposure Prophylaxis (Lenacapavir), will be offered free of charge in health facilities, in the select first priority counties for prevention purposes," the statement read.
This clarification comes days after Kenya received 21,000 starter doses of the injectable HIV prevention drug for the initial roll-out in priority counties.
In a statement on Wednesday, February 18, Health Cabinet Secretary Aden Duale said the country is also expecting additional supplies in the coming weeks.
Read More
"Kenya has received 21,000 starter doses of Lenacapavir for the initial roll-out in priority counties. We also expect an additional 12,000 continuation doses by April, to ensure that those who start the prevention injections can continue without interruption," he said.

Duale further revealed that more support has been secured from the United States government to boost access to the prevention drug.
"In addition, the United States Government has committed to support Kenya with a further 25,000 doses, which will strengthen the national roll-out and increase access for more Kenyans," he added.
The Ministry of Health said the vaccine will be introduced in phases under the guidance of national HIV data and preparedness assessments.
"The Ministry of Health, through the National AIDS and STI Control Programme (NASCOP), is implementing Lenacapavir through a phased, well-planned approach guided by national HIV data, county readiness, and the Kenya PrEP Operational Plan 2025, to ensure that the roll-out is safe, efficient, and sustainable," the CS noted.
According to Duale, the first phase of the programme will begin in early March and will target counties with high HIV burden before expanding countrywide.
"The first phase of implementation will begin in early March, covering 15 counties, followed by a second phase covering another 15 counties, and a final phase covering the remaining 17 counties, ensuring nationwide access over time.
"The first 15 counties selected for the initial roll-out are: Mombasa, Kilifi, Machakos, Nairobi, Kajiado, Nakuru, Uasin Gishu, Kakamega, Busia, Siaya, Kisumu, Migori, Homa Bay, Kisii, and Kiambu," he noted.
Duale assured Kenyans that systems have been put in place to integrate the injectable prevention drug into existing health infrastructure and to support long-term sustainability.
"The Ministry assures the public that Lenacapavir will be integrated into existing health systems, including KEMSA distribution channels, and that NASCOP has strengthened monitoring tools to track use, safety, and commodity management. Kenya is also developing a resource mobilization plan to support long-term national scale-up beyond the initial partner-supported phase," he concluded.
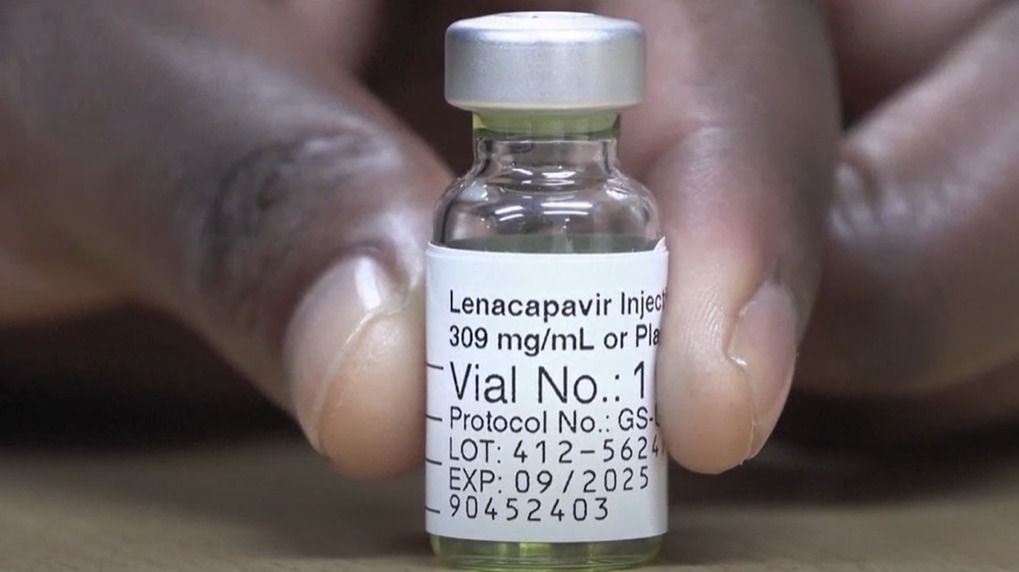



-1771580002.png)

-1771575482.png)
